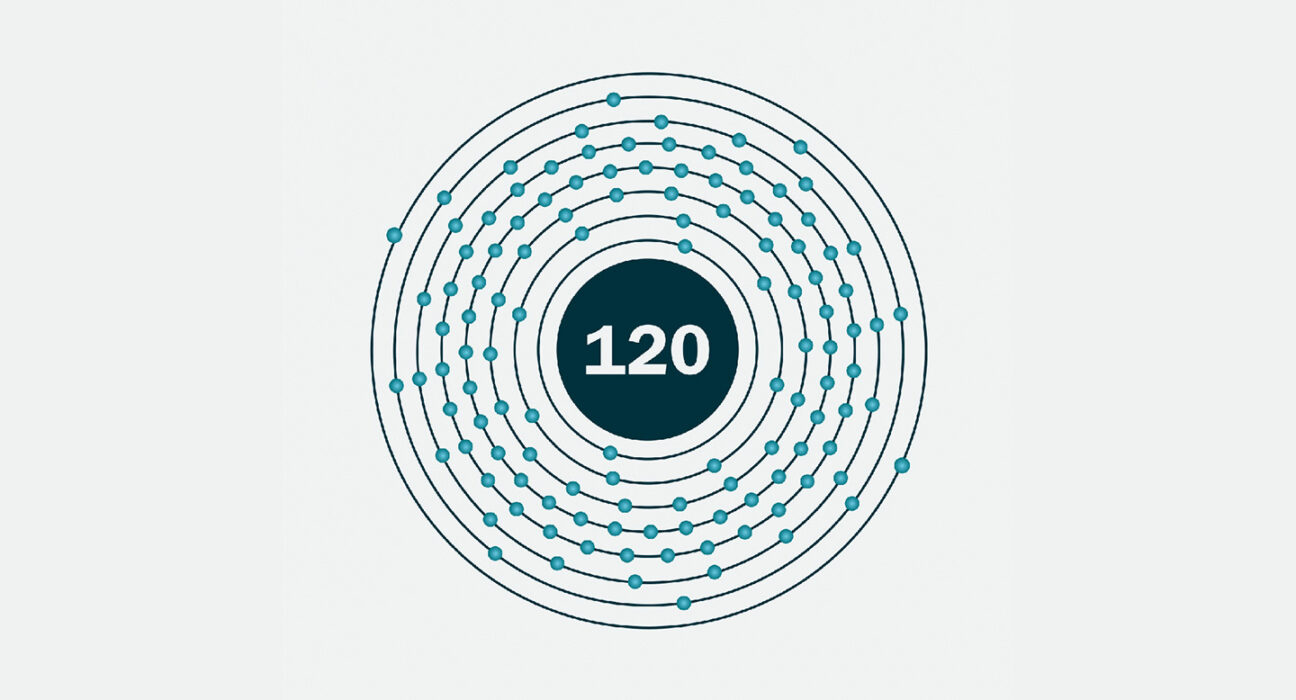
atom: The basic unit of a chemical element. Atoms are made up of a dense nucleus that contains positively charged protons and uncharged neutrons. The nucleus is orbited by a cloud of negatively charged electrons.
atomic: Having to do with atoms, the smallest possible unit that makes up a chemical element.
calcium: A chemical element and alkali metal common in minerals of the Earth’s crust and in sea salt. It is also found in bone mineral and teeth, and can play a role in the movement of certain substances into and out of cells.
chemical: A substance formed from two or more atoms that unite (bond) in a fixed proportion and structure. For example, water is a chemical made when two hydrogen atoms bond to one oxygen atom. Its chemical formula is H2O. Chemical also can be an adjective to describe properties of materials that are the result of various reactions between different compounds.
decay: (for radioactive materials) The process whereby a radioactive isotope — which means a physically unstable form of some element — sheds energy and subatomic particles. In time, this shedding will transform the unstable element into a slightly different but stable element. For instance, uranium-238 (which is a radioactive, or unstable, isotope) decays to radium-222 (also a radioactive isotope), which decays to radon-222 (also radioactive), which decays to polonium-210 (also radioactive), which decays to lead-206 — which is stable. No further decay occurs. The rates of decay from one isotope to another can range from timeframes of less than a second to billions of years.
element: A building block of some larger structure. (in chemistry) Each of more than one hundred substances for which the smallest unit of each is a single atom. Examples include hydrogen, oxygen, carbon, lithium and uranium.
forge: (verb) To shape metals under heat and/or pressure, or (colloquially) to form one element from another under the intense heat and pressure inside stars.
hydrogen: The lightest element in the universe. As a gas, it is colorless, odorless and highly flammable. It’s an integral part of many fuels, fats and chemicals that make up living tissues. It’s made of a single proton (which serves as its nucleus) orbited by a single electron.
ion: (adj. ionized) An atom or molecule with an electric charge due to the loss or gain of one or more electrons. An ionized gas, or plasma, is where all of the electrons have been separated from their parent atoms.
neutron: A subatomic particle carrying no electric charge that is one of the basic pieces of matter. Neutrons belong to the family of particles known as hadrons.
nucleus: Plural is nuclei. (in physics) The central core of an atom, containing most of its mass.
particle: A minute amount of something.
physics: The scientific study of the nature and properties of matter and energy. Classical physics is an explanation of the nature and properties of matter and energy that relies on descriptions such as Newton’s laws of motion. Quantum physics, a field of study that emerged later, is a more accurate way of explaining the motions and behavior of matter. A scientist who works in such areas is known as a physicist.
proton: A subatomic particle that is one of the basic building blocks of the atoms that make up matter. Protons belong to the family of particles known as hadrons.
radioactive: An adjective that describes unstable elements, such as certain forms (isotopes) of uranium and plutonium. Such elements are said to be unstable because their nucleus sheds energy that is carried away by photons and/or one or more subatomic particles. This emission of energy is by a process known as radioactive decay.
tactic: An action or plan of action to accomplish a particular feat.